[:en]
Environmentally friendly synthetic methods based on the use of polymers.
Modern synthetic chemistry needs to become more efficient and clean and, for this reason, the minimization of waste and cumbersome purification protocols is a very important goal.
We are interested in the synthesis of new functionalized polymers and their application in sustainable synthetic chemistry. These macromolecular scaffolds can be used to anchor reagents or catalysts so they can be more easily recovered and separated and, ideally, reused multiple times.
For that purpose we have developed polymerization and post-polymerization functionalization methods to synthesize robust polymers based on norbornene derivatives. Some norbornene polymerization routes afford an all-aliphatic polymer backbone, thermally and chemically resistant, well suited for a support material.
New polynorbornenes with aliphatic backbone:
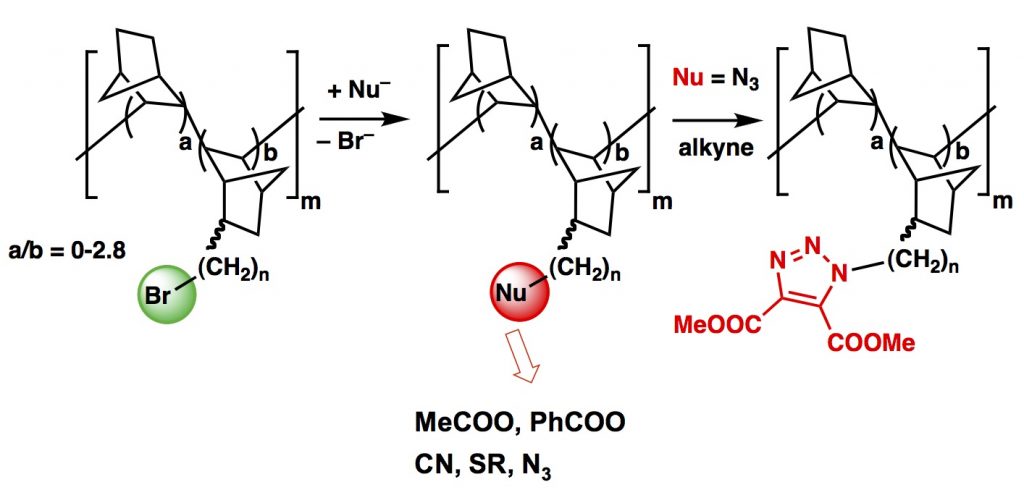
Our work is centered on vinylic addition polynorbornenes (VA-PNBs), a type of polymers that keep 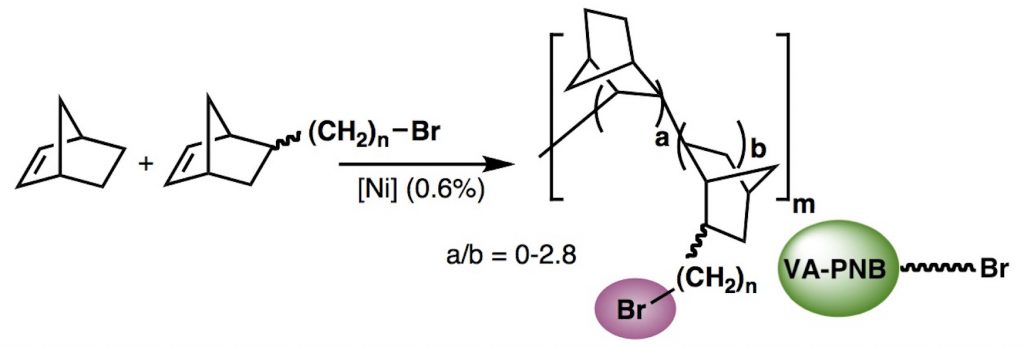 the aliphatic bicyclic structure of norbornene in the polymer and are very resistant both thermally and chemically.
the aliphatic bicyclic structure of norbornene in the polymer and are very resistant both thermally and chemically.
The direct polymerization of functionalized norbornenes is not easy, so we have developed brominated VA-polynorbornenes that can be further functionalized by a variety of postpolymerization reactions to introduce the desired supported moiety.
S. Martínez-Arranz, A. C. Albéniz, P. Espinet. Macromolecules 2010, 43, 7482.
S. Martínez-Arranz, E. Sánchez-Pérez, J. A. Molina de la Torre, I. Pérez-Ortega, A. C. Albéniz. RSC Advances, 2016, 6, 105878-105887. DOI:0.1039/c6ra23123c
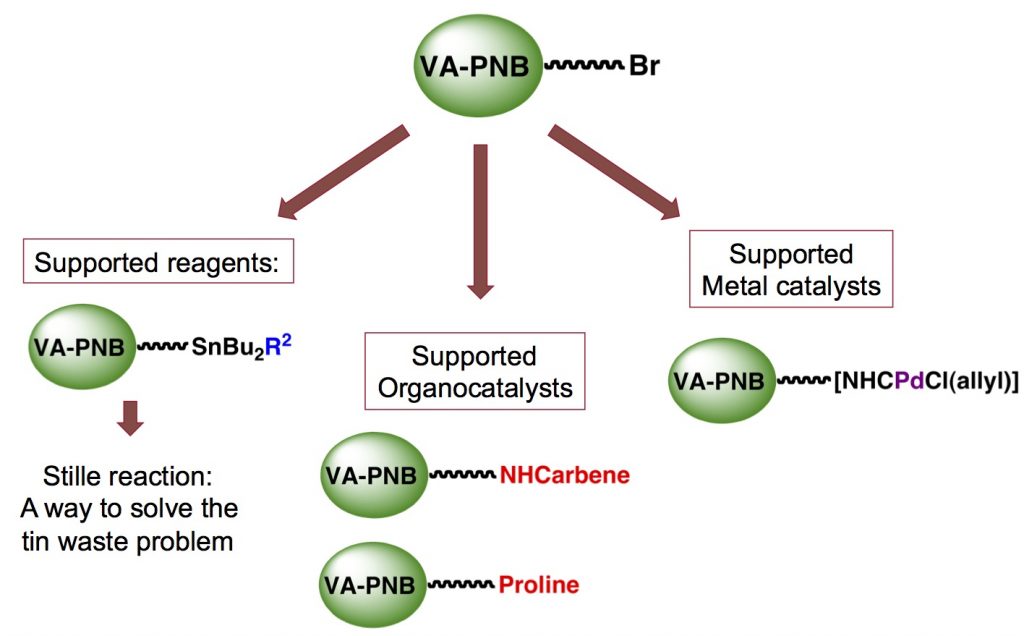
All-aliphatic polynorbornenes (VA-PNBs), a good support for reagents or catalysts:
 A clean Stille reaction:
A clean Stille reaction:
We have synthesized stannylated polymers to be used as recyclable reagents in the Stille reaction and other processes that use organotin reagents and face the problem of the generation of organotin toxic byproducts. Our work is centered on vinylic addition polynorbornenes but we have also used other polymeric scaffolds (polyfluorenes, polyphosphazenes) for this purpose.
N. Carrera, E. Gutiérrez, R. Benavente, M. M. Villavieja, A. C. Albéniz, P. Espinet. ”Stannylated Polynorbornenes as New Reagents for a Clean Stille Reaction” Chem. Eur. J. 2008, 14, 10141.
S. Martínez-Arranz, D. Presa-Soto, G. A. Carriedo, A. Presa Soto, A. C. Albéniz. “Polyphosphazenes for the Stille reaction: A new type of recyclable stannyl reagents” Dalton Trans. 2016, 45, 2227-2236. DOI: 10.1039/C5DT02670A.
The Stille reaction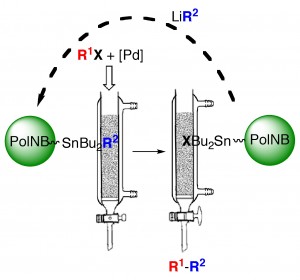 can be carried out in a batch way using immobilized tin reagents with a very simple workup and very low tin contamination in the products (15 ppm). The reagent can be regenerated and reused multiple times.
can be carried out in a batch way using immobilized tin reagents with a very simple workup and very low tin contamination in the products (15 ppm). The reagent can be regenerated and reused multiple times.
S. Martínez-Arranz, N. Carrera, A. C. Albéniz, P. Espinet, A. Vidal Moya. “Batch Stille Coupling with Insoluble and Recyclable Stannylated Polynorbornenes” Adv. Synth. & Cat. 2012, 354, 3551-3560. DOI: 10.1002/adsc.201200624
Supported organocatalysts.
VA-PNBs are very useful polymeric supports for organocatalysis. Two different types of catal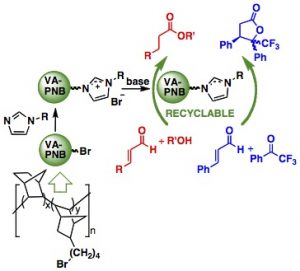 ysts were anchored and tested leading to fully recyclable systems.
ysts were anchored and tested leading to fully recyclable systems.
N-Heterocyclic carbenes supported on VA-PNBs are good catalysts for umpolung organic transformations, recyclable and can be recovered as imidazolium salts and stored indefinitely for further use:
J. A. Molina de la Torre, A. C. Albéniz, ChemCatChem, 2014, 6, 3547-3552. DOI:10.1002/cctc.201402767
Asymmetric aldol reactions in water/solvent mixtures were performed using proline-derivatives supported on VA-PNBs as catalysts, with excellent results as far as selectivity and recyclability:
I. K. Sagamanova, S. 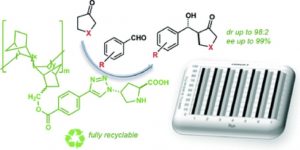 Sayalero, S. Martínez-Arranz, A. C. Albéniz, M. A. Pericás. Catal. Sci. Technol. 2015, 5, 754-764. DOI:10.1039/C4CY01344A
Sayalero, S. Martínez-Arranz, A. C. Albéniz, M. A. Pericás. Catal. Sci. Technol. 2015, 5, 754-764. DOI:10.1039/C4CY01344A
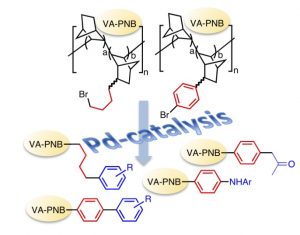
A palladium reservoir on polymer for use in catalysis.
Polymer-supported metal complexes have been used in catalysis with the aim of bringing together the advantages of both homo- and heterogenous catalysis. This has met some success, but the mechanisms of the actual catalysis are far from obvious in many cases.
A vinylic addition poly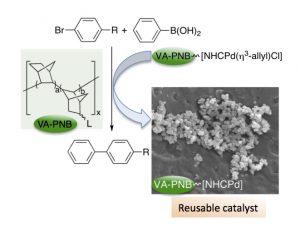 norbornene (VA-PNB) supported NHC palladium complex can used as precatalyst in C-C cross coupling reactions and it is reusable. We have studied the system in some detail and found out that after the first use, the polymer contains Pd aggregates (about 65 nm). A small amount of palladium (a few ppms) is released from those aggregates and it is enough to catalyze a Suzuki or a Negishi reaction in a homogeneous way. The polymer is a reservoir of palladium that can be used multiple times.
norbornene (VA-PNB) supported NHC palladium complex can used as precatalyst in C-C cross coupling reactions and it is reusable. We have studied the system in some detail and found out that after the first use, the polymer contains Pd aggregates (about 65 nm). A small amount of palladium (a few ppms) is released from those aggregates and it is enough to catalyze a Suzuki or a Negishi reaction in a homogeneous way. The polymer is a reservoir of palladium that can be used multiple times.
J. A. Molina de la Torre, A. C. Albéniz. ChemCatChem, 2016, 8, 2241-2248. DOI: 10.1002/cctc.201600194.
Supported trispyrazolylborates for catalysis with copper.
We have managed to anchor other functionalities on VA-PNBs such as diimines (Eur. J. Inorg. Chem. 2017, DOI: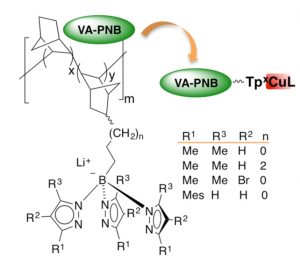 10.1002/ejic.201700323) or the tridentate trispyrazolylborate ligand (Tp). In collaboration with the group of P. J. Perez at the CIQSO-University of Huelva, we have developed a recyclable TpCu catalyst for carbene or nitrene transfer reactions.
10.1002/ejic.201700323) or the tridentate trispyrazolylborate ligand (Tp). In collaboration with the group of P. J. Perez at the CIQSO-University of Huelva, we have developed a recyclable TpCu catalyst for carbene or nitrene transfer reactions.
J. A. Molina de la Torre, I. Pérez-Ortega, A. Beltrán, M. R. Rodríguez, M. M. Díaz-Requejo, P. J. Pérez, A. C. Albéniz. Trispyrazolylborate Ligands Supported on Vinylic Addition Polynorbornenes and their Copper Derivatives as Recyclable Catalysts. Chem. Eur. J. 2019, 25, 556-563. DOI: 10.1002/chem.201803852
[:es]
Metódos sintéticos respetuosos con el medio ambiente basados en el uso de polímeros.
La química sintética moderna necesita ser más limpia y eficaz y, por esta razón, minimizar la generación de residuos y evitar protocolos de purificación complejos, es un objetivo primordial.
Estamos interesados en la síntesis de nuevos polímeros funcionalizados y su aplicación a una química sintética sostenible. Estos esqueletos macromoleculares se pueden usar para anclar reactivos o catalizadores, se modo que se puedan recuperar y separar con facilidad e, idealmente, usarse muchas veces.
Con este propósito, hemos desarrollado métodos de polimerización y funcionalización post-polimerización para sintetizar polímeros robustos basados en derivados de norborneno. Algunas vías de polimerización de norborneno dan lugar a polímeros con un esqueleto completamente alifático, térmica y químicamente estables, muy adecuados como material de soporte.
Nuevos polinorbornenos de esqueleto alifático.
Nuestro trabajo se centra en el uso de polinorbornenos de adición vinílica (VA-PNBs), un tipo de polímeros que mantiene la estructura bicíclica del norborneno en el polímero y son muy resistentes tanto química como térmicamente. 
La polimerización directa de norbornenos funcionalizados no es fácil, por lo que hemos desarrollado polinorbornenos con grupos alquil o arilbromo que se pueden funcionalizar mediante una variedad de reacciones de postpolimerización para introducir el grupo que se quiera soportar.
S. Martínez-Arranz, A. C. Albéniz, P. Espinet. Macromolecules 2010, 43, 7482.
S. Martínez-Arranz, E. Sánchez-Pérez, J. A. Molina de la Torre, I. Pérez-Ortega, A. C. Albéniz. RSC Advances, 2016, 6, 105878-105887. DOI:0.1039/c6ra23123c
Polinorbornenos alifáticos (VA-PNBs), un buen soporte de reactivos o catalizadores:
 Una reacción de Stille limpia:
Una reacción de Stille limpia:
Hemos sintetizados polímeros estannilados que se pueden usar como reactivos reciclables en la reacción de Stille y otros procesos que usan derivados organometálicos de estaño, y que se enfrentan al problema de la generación de subproductos organoestannilados tóxicos. Nuestro trabajo se centra en el uso de polinorbornenos de adición vinílica, pero también hemos usado otros esqueletos poliméricos para este propósito (polifluorenos, polifosfacenos).
N. Carrera, E. Gutiérrez, R. Benavente, M. M. Villavieja, A. C. Albéniz, P. Espinet. ”Stannylated Polynorbornenes as New Reagents for a Clean Stille Reaction” Chem. Eur. J. 2008, 14, 10141./span>
S. Martínez-Arranz, D. Presa-Soto, G. A. Carriedo, A. Presa Soto, A. C. Albéniz. “Polyphosphazenes for the Stille reaction: A new type of recyclable stannyl reagents” Dalton Trans. 2016, 45, 2227-2236. DOI: 10.1039/C5DT02670A./span>
La reacción de Stille se puede llevar a cabo mediante un procedimiento tipo batch usando el reactivo polimérico de estaño inmovilizado, con un procedimiento experimental muy sencillo y muy baja contaminación de estaño en los productos (15 ppm). El reactivo puede ser regenerado y reutilizado.
(15 ppm). El reactivo puede ser regenerado y reutilizado.
S. Martínez-Arranz, N. Carrera, A. C. Albéniz, P. Espinet, A. Vidal Moya. “Batch Stille Coupling with Insoluble and Recyclable Stannylated Polynorbornenes” Adv. Synth. & Cat. 2012, 354, 3551-3560. DOI: 10.1002/adsc.201200624/span>
Organocatalizadores soportados.
Los VA-PNBs son soportes útiles de organocatalizadores. Se han anclado dos tipos diferentes de catalizadores y se han probado, demostrando que son sistemas completamente reciclables. 
Carbenos N-Heterocíclicos soportados en VA-PNBs son buenos catalizadores en transformaciones orgánicas de tipo umpolung, son reciclables y se pueden recuperar como sales de imidazolio y almacenar de forma indefinida hasta que necesiten volver a usarse.
J. A. Molina de la Torre, A. C. Albéniz, ChemCatChem, 2014, 6, 3547-3552. DOI:10.1002/cctc.201402767
Las reacciones asimétricas de tipo aldol en mezclas agua/disolvente se pueden catalizar con derivados de prolina anclados sobre VA-PNBs con excelentes resultados tanto en cuanto a selectividad como reciclabilidad.
I. K. Sagamanova, S.  Sayalero, S. Martínez-Arranz, A. C. Albéniz, M. A. Pericás. Catal. Sci. Technol. 2015, 5, 754-764. DOI:10.1039/C4CY01344A
Sayalero, S. Martínez-Arranz, A. C. Albéniz, M. A. Pericás. Catal. Sci. Technol. 2015, 5, 754-764. DOI:10.1039/C4CY01344A
Una fuente de paladio sobre un polímero para su uso en catálisis.
Se han usado complejos metálicos soportados en polímeros con el objetivos de conseguir aunar las ventajas que presentan tanto la catálisis homogénea como la heterogénea. Esta aproximación ha tenido algún éxito aunque en muchas ocasiones los mecanismos que operan en estos tipos de catálisis no son obvios ni fáciles de estudiar.
Hemos preparado y usado un complejo de paladio con un carbeno NHC como ligando soportado sobre un polinorborneneo de adición vinílica (VA-PNB) como precatalizador en reacciones de acoplamiento cruzado C-C que se puede reutilizar. Hemos estudiado el sistema con detalle y hemos encontrado que, después del primer uso, el polímero contiene agregados de paladio sobre el mismo (alrededor de 65 nm). Una pequeña cantidad de paladio (unas pocas ppms) se libera de dichos agragados en cada reacción, cantidad suficiente para catalizar reacciones de Suzuki o Negishi en fase homogénea. El polímero actúa como una fuente de paladio que puede ser reutilizada en múltiples ocasiones de forma eficaz.
(VA-PNB) como precatalizador en reacciones de acoplamiento cruzado C-C que se puede reutilizar. Hemos estudiado el sistema con detalle y hemos encontrado que, después del primer uso, el polímero contiene agregados de paladio sobre el mismo (alrededor de 65 nm). Una pequeña cantidad de paladio (unas pocas ppms) se libera de dichos agragados en cada reacción, cantidad suficiente para catalizar reacciones de Suzuki o Negishi en fase homogénea. El polímero actúa como una fuente de paladio que puede ser reutilizada en múltiples ocasiones de forma eficaz.
J. A. Molina de la Torre, A. C. Albéniz. ChemCatChem, 2016, 8, 2241-2248. DOI: 10.1002/cctc.201600194.
Supported trispyrazolylborates for catalysis with copper.
Hemos conseguido anclar otros ligandos sobre VA-PNBs como diiminas (Eur. J. Inorg. Chem. 2017, DOI: 10.1002/ejic.201700323) o ligando tridentado de tipo trispirazolilborato (Tp). En colaboración con el grupo de P. J. Perez en el CIQSO-Universidad of Huelva, hemos desarrollas un catalizador reciclable de tipo ToCu para su uso en reacciones de transferencia de carbeno o nitreno.
10.1002/ejic.201700323) o ligando tridentado de tipo trispirazolilborato (Tp). En colaboración con el grupo de P. J. Perez en el CIQSO-Universidad of Huelva, hemos desarrollas un catalizador reciclable de tipo ToCu para su uso en reacciones de transferencia de carbeno o nitreno.
J. A. Molina de la Torre, I. Pérez-Ortega, A. Beltrán, M. R. Rodríguez, M. M. Díaz-Requejo, P. J. Pérez, A. C. Albéniz. Trispyrazolylborate Ligands Supported on Vinylic Addition Polynorbornenes and their Copper Derivatives as Recyclable Catalysts. Chem. Eur. J. 2019, 25, 556-563. DOI: 10.1002/chem.201803852