[:en,es][:es]
El entendimiento preciso del mecanismo de una reacción permite, en general, mejorar sus condiciones de aplicación y la eficacia de la misma. En reacciones catalíticas las posibilidades de comprender su mecanismo aumentan considerablemente cuando pueden aislarse algunos intermedios y estudiarse separadamente algunas etapas de las que componen el ciclo catalítico. Eso lo hacemos mediante el seguimiento espectroscópico de las reacciones y el estudio de sus cinéticas. En esta página te mostramos algunos de nuestros resultados más recientes.
Recientemente hemos encontrado un sistema que nos permite estudiar lo que sucede cuando en la reacción de Stille la transmetalación es más rápida que las isomerizaciones de los complejos de paladio implicados.
La reacción de Negishi transcurre de forma diferente cuando se emplean reactivos del tipo ZnRX o cuando se emplean reactivos ZnR2. La reacción de transmetalación es reversible, y la estereoquímica de la reacción está condicionada por la velocidad con la que se establecen los equilibrios de transmetalación-retrometalación.

De izquierda a derecha: Juan A. Casares, Ei-Ichi Negishi y Pablo Espinet en el 20 Congreso Euchems de Química Organometálica. (StAndrews, Escocia, julio de 2013)
[:en]The possibility of understanding the mechanism of catalytic reactions depends very much on the chances of isolating intermediates and to study isolately each step in the catalytic cycle. We try to do this by monitoring the reactions and spectroscopic study of its kinetics. On this page we show you some of our latest results.
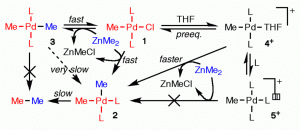
The complexity of the transmetalation step in a Pd-catalyzed Negishi reaction has been investigated by combining experiment and theoretical calculations. The reaction between trans-[PdMeCl(PMePh2)2] and ZnMe2 in THF reveal some unexpected and relevant mechanistic aspects not observed for ZnMeCl as nucleophile. The operative reaction mechanism is not the same when the reaction is carried out in the presence or in the absence of an excess of phosphine in the medium. In the absence of added phosphine an ionic intermediate with THF as ligand ([PdMe(PMePh2)2(THF)]+) opens ionic transmetalation pathways.
J. Am. Chem. Soc, 2011, 133, 13519–13526.
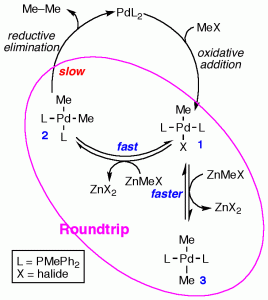
During the sutdy of the Negishi reaction we have also found that Pd takes a roundtrip before coupling: The large rate difference of the slow reductive elimination and the fast transmetalation-retrotransmetalation steps brings Pd to visit many times its complexes 3, 2, and 1, before coupling from 2 takes place. This fast recurrence of transformations offers many opportunities for the formation of undesired homocoupling products in Negishi reactions, via statistically accessible “mistaken” retrotransmetalation events in the multitude of roundtrip processes preceding the slower irreversible reductive elimination of the coupling product.
Chem. Eur. J., 2010, 16, 8596-8599.
We recently found a system that allows us to study what happens when during the Stille reaction the transmetallation is faster than the isomerization reactions of the palladium complexes involved.